Abstract
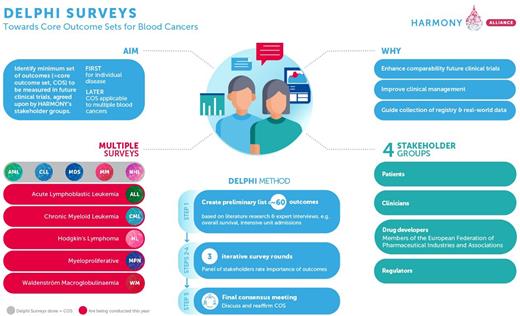
Introduction: The definition of a core outcome set (COS), which represents an agreed set of outcomes for each hematological malignancy (HM) may improve the interpretation and comparability of clinical trials, especially if a respective COS addresses the needs of all stakeholders including patients, clinicians, industry, as well as regulators/HTA bodies. In accordance, HARMONY - the Healthcare Alliance for Resourceful Medicine Offensive against Neoplasms in Hematology -has made it its mission to develop COS for HMs.
Methods: For COS definition and consensus finding the Delphi method was used including all stakeholder groups (patients, clinicians, industry, and regulators/HTA bodies) based on comprehensive outcome lists generated for each of the HMs using published reports, literature and available guidelines. Conditions and criteria how to define the COS were defined in study protocols, which were made publicly available for each HM (Delphi Projects - HARMONY Alliance (harmony-alliance.eu). Classical Delphi surveys were conducted for acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), myelodysplastic syndrome (MDS), multiple myeloma (MM), and chronic lymphatic leukemia (CLL). Surveys using a modified virtual meeting approach were conducted for chronic myeloid leukemia (CML), myeloproliferative neoplasms (MPN), Hodgkin lymphoma (HL) and Waldenström's macroglobulinemia (WM) as using the classical Delphi approach we had experienced high drop-out rates in each of the stakeholder groups during the COVID pandemic. In accordance, for the HMs subject to HARMONY PLUS we have developed a "Delphi Hackathon" approach comprised of two virtual Delphi rounds during which members of all stakeholder groups did participate in the surveys in parallel. For both the classical Delphi surveys and the Delphi Hackathons consensus meeting were conducted to discuss the results based on the previously defined "consensus-in" cut-offs. For outcomes especially valuable to patients, a special label "patient-important criterion" was implemented. Finally, following a bottom-up-approach, i.e. starting with the definition of COS for each individual HM, an overarching COS was created on the basis of the individual HM survey results.
Results: For the HARMONY Delphi surveys a total of 365 individuals participated including 177 patients/patient advocates (48%), 126 clinicians (35%), 46 EFPIA/industry members (13%), and 16 regulators/members of HTA bodies (4%). In summary, for the HARMONY HMs 11 out of 59 outcomes met the consensus-in criterion in AML, 8 out of 61 in NHL, 12 out of 51 in MDS, 12 out of 58 in MM, and 17 out of 54 in CLL. For the HARMONY PLUS Delphi surveys, 161 persons participated in total including 20 patients/patient advocates (12%), 93 clinicians (58%), 39 EFPIA/industry members (24%), and 9 regulators/members of HTA bodies (6%). Compared to the primary Delphi approach the dropout rates were significantly lower (9% vs 46%, respectively) and the duration of the Delphi from start until the consensus meeting much shorter (3 months vs 18 months, respectively). Individual HM COS results showed that it is harder to mobilize different stakeholder groups depending on the nature of the disease, e.g. easier to attract patients/patient advocates in HMs with more chronic disease course, whereas it is easier to get physician impact in the more aggressive disease with unfavorable outcome. Overall, COS contained outcomes reflecting all outcome groups, i.e. PRO, use of healthcare resources, type of event, time to event, clinical parameters and safety concerns. Detailed results will be provided for all HMs during the ASH meeting.
Conclusion: To the best of our knowledge, this is the first multidisciplinary approach to define COS for HMs including the views of all important stakeholder groups with a special focus on patients' needs. The COS results of this HARMONY/HARMONY PLUS task will not only allow to compare findings across different trials within a distinct HM subgroup, but also to more easily compare results across different HMs. Following some final expert panel meeting to be held during the HARMONY General Assembly Meeting in October 2022, our results will form the basis for future patient management improvements.
Disclosures
Morgan:AstraZenca, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Mundipharma, Novartis, Oncopeptides, Pfizer, Roche, Sanofi, Takeda, Gilead: Other: unrestricted core funding. Wintrich:Amgen: Other: Grant funding to the organization; Abbvie: Other: Grant funding to the organization; AstraZeneca: Other: Grant funding to the organization; Adaptive: Other: Grant funding to the organization; Astellas: Other: Grant funding to the organization; Autolus: Other: Grant funding to the organization; BMS: Other: Grant funding to the organization; Daiichi Sankyo: Other: Grant funding to the organization; Gilead/Kite: Other: Grant funding to the organization; Glycostem: Other: Grant funding to the organization; Incyte: Other: Grant funding to the organization; Janssen: Other: Grant funding to the organization; Kura Oncology: Other: Grant funding to the organization; Jazz: Other: Grant funding to the organization; Novartis: Other: Grant funding to the organization; Otsuka: Other: Grant funding to the organization; Pfizer: Other: Grant funding to the organization; Roche: Other: Grant funding to the organization; Servier: Other: Grant funding to the organization; Takeda: Other: Grant funding to the organization. York:Gilead: Other: Grant funding to the organization; Lily: Other: Grant funding to the organization; Beigene: Other: Grant funding to the organization; AstraZeneca: Other: Grant funding to the organization; Janssen: Other: Grant funding to the organization; Abbvie: Other: Grant funding to the organization. Sonneveld:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding. Santini:AbbVie: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Srvier: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria. Hochhaus:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Barbui:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Borchmann:Miltenyi Biotec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novarts: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Buske:Roche/Genentech, Janssen, Celltrion, MSD, Pfizer, and Amgen: Research Funding; Roche, Janssen, BeiGene, Celltrion, AbbVie, Pfizer, and Gilead Sciences: Speakers Bureau; Gilead Sciences, Janssen, Roche, Pfizer, BeiGene, Celltrion, AbbVie, Incyte, Regeneron, Morphosys, and Novartis: Consultancy; Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, Incyte, AbbVie, Gilead Sciences, Celltrion, Morphosys, and Regeneron: Honoraria. Guillevic:BMS: Current Employment. Calado:Novartis: Current Employment. Sanz:La Hoffman Roche Ltd.: Other: Advisor or review panel participant; Novartis Oncology: Consultancy; Celgene Corporation: Consultancy; Janssen Pharmaceuticals, Inc.: Other: Teaching and Speaking; Abbvie Pharmaceuticals: Other: Advisor or review panel participant; takeda: Honoraria; Helsinn: Honoraria, Other: Advisor or review panel participant; Takeda Pharmaceuticals Ltd: Other: Advisor or review panel participant. Schulze-Rath:Bayer Pharma AG: Current Employment, Current equity holder in private company. Bullinger:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Oncology: Research Funding; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal